Roadshow
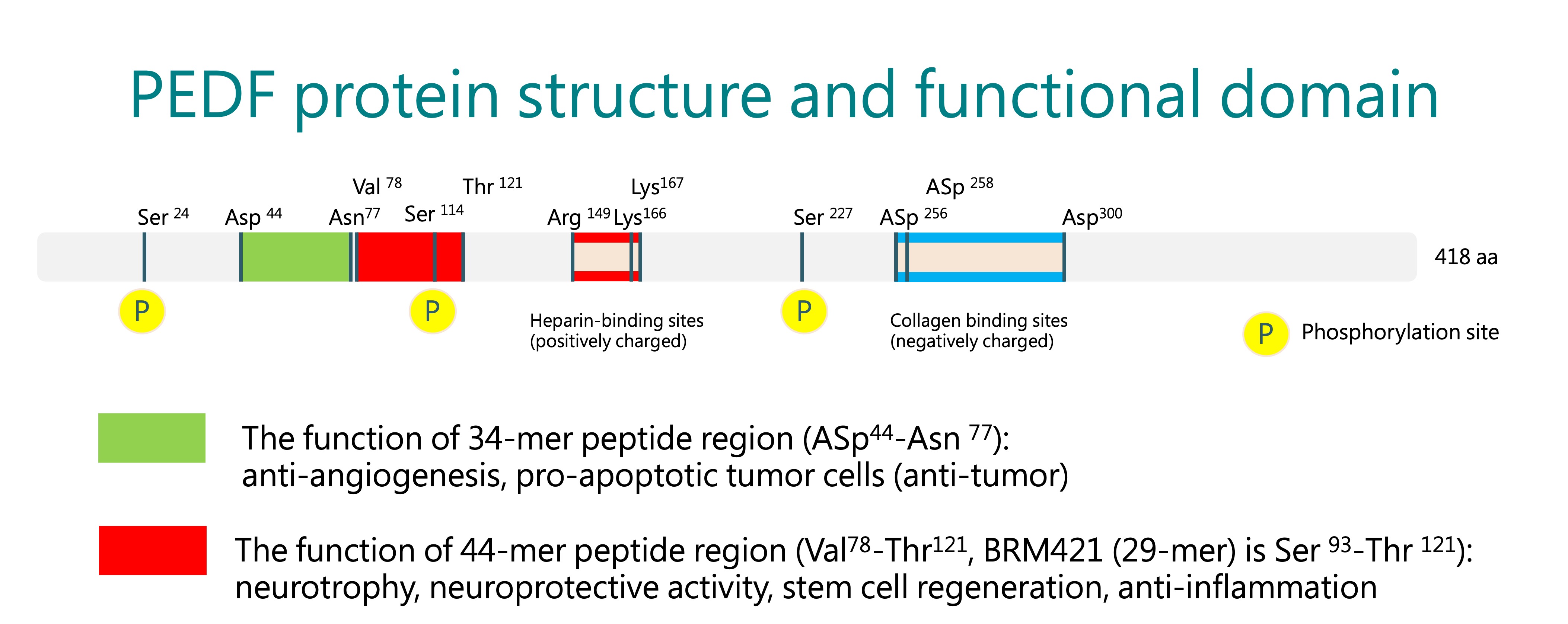
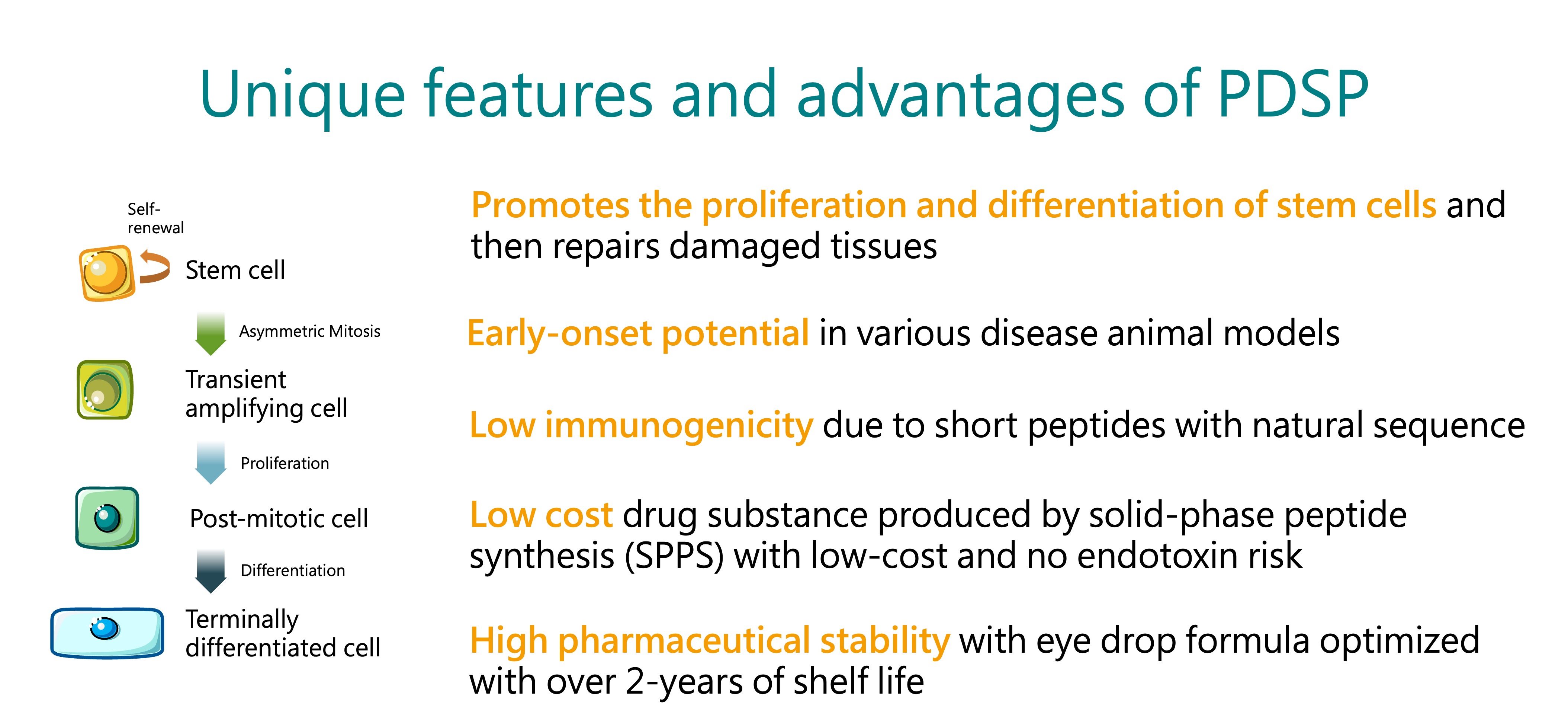
PEDF-Derived Short Peptides, PDSP
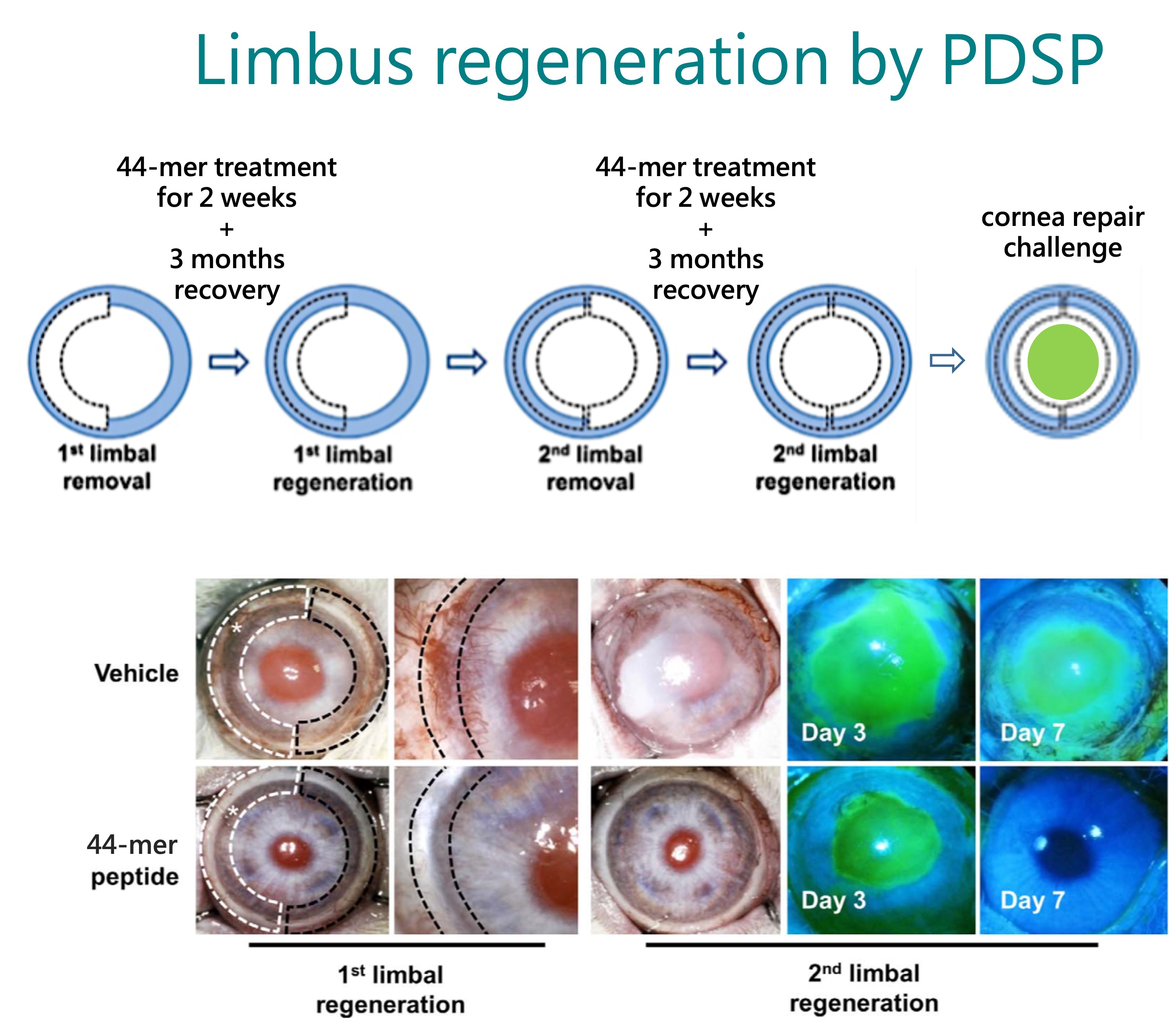
BRIM’s core technology platform is based on the Pigment epithelium-derived factor (PEDF)-Derived Short Peptides, PDSP. It was licensed from the Mackay Memorial Hospital in 2015, and BRIM completed the IND filing for BRM421 treating dry eye disease within 18 months after the technology transfer was completed. BRM421 is the first-in-class innovative drug that the US FDA agreed the first-in-human study to be a phase 2 clinical trial design to skip the phase 1 trial. Now, the planning of the second phase 3 trial of BRM421 is underway.
Besides in ophthalmology, PDSP can also be used in other indications. Through the efforts of our RD team, BRIM is actively developing additional applications for PDSP to expand the pipeline and global patent portfolio. In the hope of creating value with new drug development, BRIM strives to provide high-quality, safe, effective, and affordable medicines for patients in need.

- Established 2013
- Company BRIM Biotechnology, Inc.
- Telephone +886-2-2659-8586
- Address 8F, No.1, Alley 30, Lane 358, Ruiguang Rd.,Neihu Dist., Taipei 11492, Taiwan
BRIM translates research into clinical assets with the highest potential to positively impact patient lives. BRIM primarily targets highly novel projects with new molecular entities and niche platforms, targeting diseases where there are unmet medical needs. This involves, in-licensing from, or partnering with, academic institutions and biotech or pharma companies, both locally and internationally.
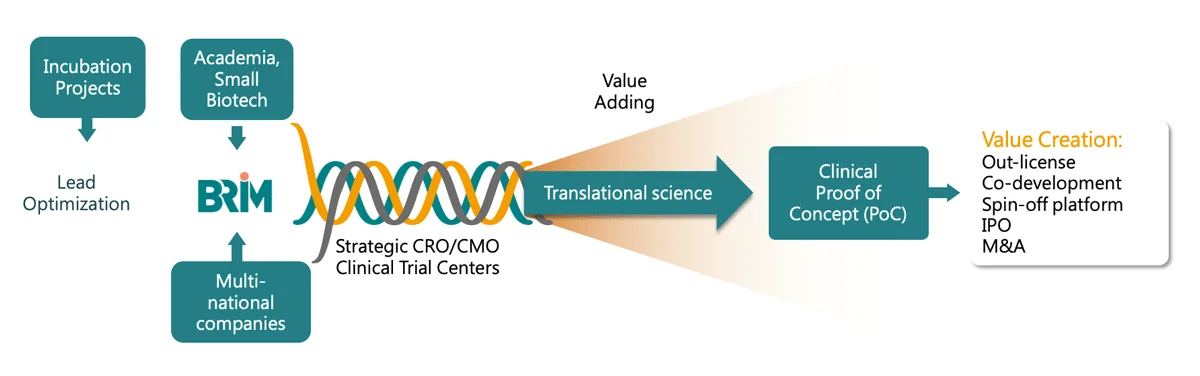
De-risking is the essence of value creation, delivered through our scientific expertise and our virtual team model. An internal integrated team of project management professionals and translational science experts provide expert input and operational leadership while outsourcing research activities to external entities such as research institutes, CROs, and CMOs.
BRIM’s model of connectivity, shared knowledge and scientific excellence means development times and costs are dramatically reduced. Upon reaching the clinical proof of concept (PoC), we out-license or partner with the best-suited third party to continue development through market approvals and commercialization, maximizing profit through royalties, milestones, or profit sharing. Our efficient business model delivers maximum ROI and is not limited by the traditional pharma R&D approach.
All drug development activities are executed according to global regulatory requirements and standards. Clinical trials are conducted to meet requirements for regulatory submissions to the US FDA through the help of CROs, CMOs, CDMOs, and partnership networks (e.g., hospitals, KOLs, government-sponsored agencies, research institutes,…etc.). BRIM’s translational science expertise enables rapid drug development and regulatory progression while ensuring high-quality standards.