Roadshow
ON101 (FESPIXON®)
ON101 is a first-in-class new drug targeting the subsets of macrophages to promote healing of chronic wounds. It has been demonstrated with superior efficacy compared to standard care dressing (p=0.0001) in treating diabetic foot ulcers in an international RCT completed in the US, Taiwan, and China. It has been approved and marketed in Taiwan, Singapore, Malaysia, and China. Global market expansion is underway to benefit the worldwide patients suffering from chronic wounds.
Join us at BIO 2024, June 3-6 in San Diego, where Oneness Biotech Co., Ltd. will be showcasing our cutting-edge solutions and services. We can't wait to connect with you to discuss how we're shaping the future of biotechnology. Find us at Booth 1917. See you there! #BIO2024#TAIWAN PAVILION

ON101 (FESPIXON®)
Indications: Diabetic Foot Ulcers (DFU)
Mechanism of Action:Re-balances the expression of M1/M2 macrophages in chronic wounds by inhibiting inflammation and initiating ADPC-driven cascade for tissue repair
- Reduces pro-inflammatory M1 macrophages, stimulates adipocyte progenitor cells to secrete GCSF and CXCL3, increasing pro-healing/pro-remodeling M2a/M2c macrophages. (JID Innovations, 2022)
- Inhibits inflammation through inhibition of the NLRP3 inflammasome activation pathway. (Frontiers in Pharmacology, 2019)
- Promotes tissue regeneration and collagen production to promote complete wound healing.
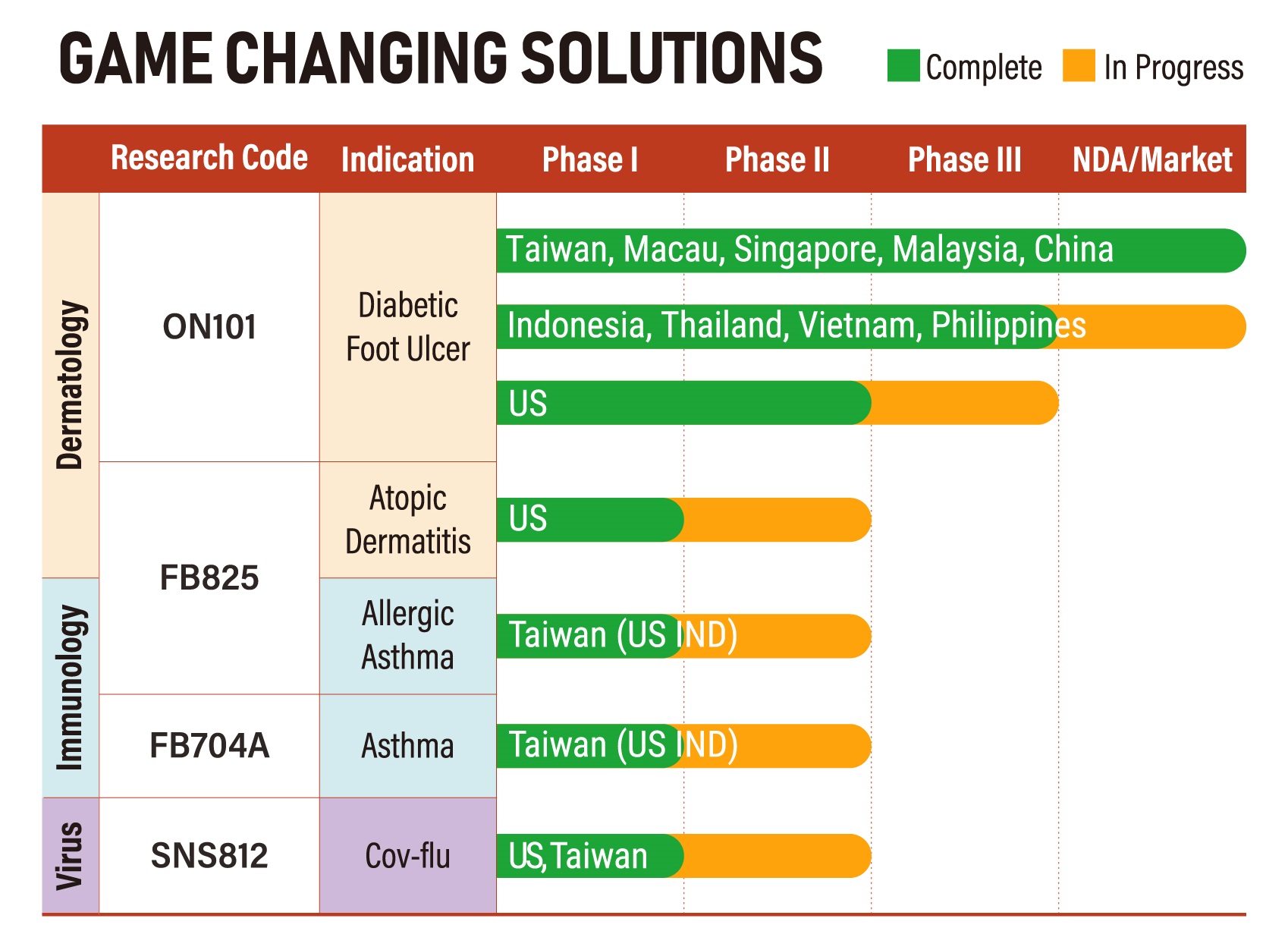
Current Status:
- Phase 3 multicenter randomized clinical trials (MRCT) was completed. ON101 has been demonstrated with 60.7% vs 35.1% (p=0.0001) in complete healing rate in 16-week treatment. A subgroup analysis on difficult-to-heal ulcers also shows the stastistical significance, consistency, and robustness of the therapeutic effect of ON101. The related data was published in the international medical journal JAMA Network Open (JAMA Network Open.2021;4(9):e2122607)
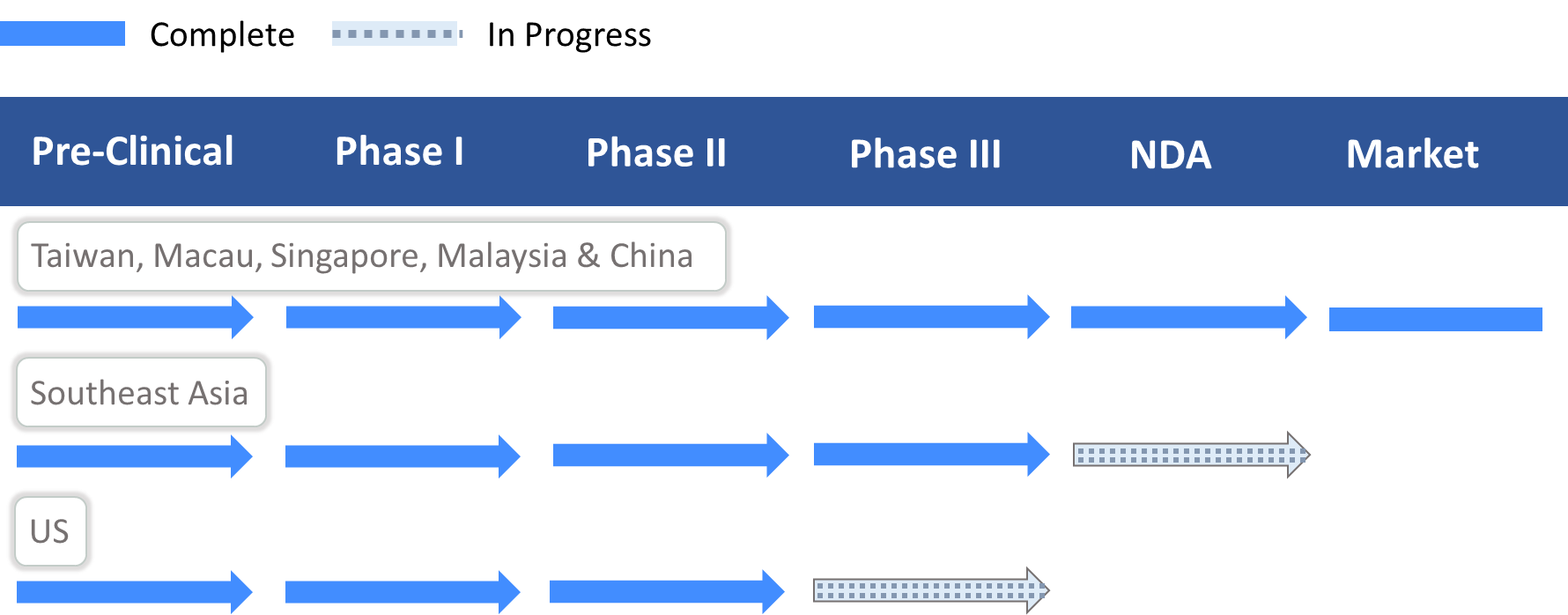
- Granted a drug approval in Taiwan, Macau, Singapore, Malaysia and China. Under NDA review in the Philippines (by the FDA Philippines, PFDA), Vietnam (by Drug Administration of Vietnam, DAV), and Indonesia (by Badan Pengawas Obat dan Makanan, BPOM).
- Granted Fast Track Designation by the US FDA.
Product Advantages:
- Effectiveness: ON101 has been clinically proven with a significant wound healing effect and can reduce the formation of hypertrophic scars.
- Cost advantage: Oneness Biotech implements a streamlined control from R&D, cultivation of the medicinal plants, production to quality control to ensure global supply capability and competitiveness.
Market Potentials:
According to a market research report by Fortune Business Insights, the global market size of diabetic foot ulcer (DFU) treatment was USD 6.6 billion in 2018, with the compound annual growth rate at 6.8%, and the market size of 2026 is estimated to be USD 11 billion. Global TAM of chronic wound management exceeds USD 22 billion.
R & D Progress:
- #Chronic Wounds
- #Surgical Wounds
- #Burns
- #Radiation Dermatitis
- #Dermatology
- #Diabetic Foot Ulcers

- Established 2008
- Company Oneness Biotech Co., Ltd.
- Telephone +886-2-2703-1098
- Email zoe.chen@onenessbio.com.tw
- Address 35F., No. 66, Sec. 1, Zhongxiao W. Rd., Zhongzheng Dist., Taipei City 100507, Taiwan
About Oneness
Founded in 2008, Oneness Biotech is a leading biotech in Taipei, Taiwan developing game-changing therapeutics in chronic dermatological and immunological disorders. It went IPO since 2011, becoming a commercial stage biotech company with its lead product being commercialized globally in 2024.
- Innovative pipelines to provide game-changing solutions:
♦ON101 : First-in-class macrophage-regulator for millions of chronic wounds - ongoing global market expansion via partnership
♦FB825 : First-in-class anti-IgE B cell mAb out-licensed to LEO Pharma for US$530 M deal since 2020
♦FB704A : anti-IL6 mAb and SNS812, siRNA targeting coronaviruses in phase 2 stage validating in-house R&D capability bringing assets from pre-clinical to clinical
- Integrated platform with full-human antibody library, pre-clinical proof-of-concept capability and PIC/s GMP certified manufacturing facilities with production capacity of 25 million tubes of ON101 for global market
- Experienced leadership team in strategy, R&D and market access.
We strengthen our collaboration with global pharma companies based on our breakthrough innovation and science. Based in Taiwan, we hope to connect the world with our game-changing solutions and make contribution to human health.